ㅤ

Article by Katelyn Zhu & Margaretha Morsink
Machine-Learning Based Drug Screen Identifies Treatment for Cardiac Fibrosis
Source Publication:
Multiscale drug screening for cardiac fibrosis identifies MD2 as a therapeutic target, Cell, 2024, Hao Zhang et al., Joseph Wu Lab
Fibrosis is the buildup of too much scar tissue in the body. It plays a major role in many diseases and can eventually lead to organ failure. Finding effective treatments has been difficult because researchers have not had enough dependable human cells to accurately test new drugs. In this study, scientists focused on cardiac fibrosis, which affects the heart. They used a type of stem cell, called an induced pluripotent stem cell, to produce heart-related fibroblasts and small tissue-like structures. This allowed them to perform a drug screen and identify a potential compound that revealed an important signaling pathway, and a possible treatment target, for reversing the scarring process.
What did these researchers do?
To establish a reliable cellular model, the researchers derived cardiac fibroblasts from induced pluripotent stem cells (iPSC-CFs), a type of stem cell capable of becoming nearly any cell type in the body and available in abundant supply. To track fibrosis-related changes, they used gene editing to label a key marker of fibroblast activation. Using this model, they screened a library of 5,000 compounds and identified Artesunate (ART) as the most effective at reducing fibrotic effects without harming cell viability. To confirm the effects of ART, the researchers tested it in several models, including human heart cells, tissues from patients with heart disease, 3D-lab grown heart tissue, and mice with heart failure. In all instances, ART reduced signs of fibrosis and helped improve heart function. While ART is known to block inflammation-related signals in immune cells, its role in heart cells was unclear. The study found that ART blocks a protein complex (called TLR4/MD2) that normally triggers fibrosis in response to tissue damage. By preventing the complex from forming, ART may stop the harmful feedback loop that would otherwise drive scar tissue buildup in the heart.
Why is this important?
Fibrosis diseases contribute to around 35% of global deaths, revealing the severity and importance of finding therapeutic approaches. There are currently no FDA-approved drugs that target cardiac fibrosis. Those that exist on the market for other fibrotic diseases exhibit toxic side effects or fail to address the full complexity of the disease. This study takes an important step toward understanding the biological pathways that drive fibrosis and identifying specific targets in the body that could help lead to effective therapies for patients.
How did the researchers do this?
The researchers used gene editing technology to tag a key fibrosis marker with Clover2, a fluorescent protein that lights up during the differentiation of myofibroblasts (MyoFBs), which are the cells responsible for scar tissue formation. The high-throughput drug screening tested 5,000 compounds. They detected Clover2 fluorescent intensity, as well as a live cell count, to see which drug best reduced fibrosis activity without simply killing all the cells. Through this, the researchers concluded ART was the most effective drug.
To ensure the effects of ART are applicable beyond iPSC-CFs, the researchers looked at human primary CFs, which they stimulated with profibrotic factors to activate signs of fibrosis. After applying ART, they saw that the drug decreased MyoFBs just like it did for the iPSC-CF lines. These 2D cell lines can’t fully capture the full scale of human tissue in the body, so they also developed a 3D engineered heart tissue (EHT) model that could measure contraction, an important part of heart function. To do this, they combined iPSC-CFs with iPSC-cardiomyocytes (CMs) to create compact tissues before stimulating it to induce profibrotic phenotype. ART not only decreased markers of fibrosis, but also improved heart function. Lastly, the researchers looked at heart failure in mice models. This perspective places ART in a more complex and physiologically relevant environment. The mice underwent transverse aortic constriction (TAC) surgery, which triggers excess fibrosis formation. Once again, ART reduced collagen deposition and showed signs of remodeling of the heart.
Finally, the researchers were able to find the underlying mechanism by which the drug helps with fibrosis.
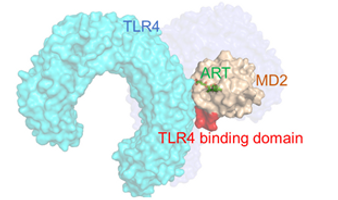
Mechanism of the drug ART that reduces fibrosis.
What comes next?
This study introduces an efficient, human-based cell system that can help identify medicines for treating fibrosis. Although the compound ART appears promising, it will likely only be useful for short-term treatment. Its long-term safety is still unknown and will require more testing. In addition, other biological pathways may also play a role in the antifibrotic effects seen in the study, not just the TLR4/MD2 pathway. Fibrosis is a complex condition that can develop from many different processes in the body, so more research is needed to find better and more effective treatment targets.
An important part of this study is the use of induced pluripotent stem cells (iPSCs). These cells can be grown in large amounts and can be turned into many different cell types, making them valuable for studying diseases and testing new drugs. Their usefulness goes beyond fibrosis: they can also be used to model conditions such as dilated cardiomyopathy, Alzheimer’s disease, and cystic fibrosis. Therefore, the use of iPSCs with a fluorescent reporter line can be used to find new drugs for other diseases as well.