ㅤ

Article by Yena Shin, Margaretha Morsink & Roberta Lock
Gene Editing Treatment for Sickle Cell Disease
Source Publication:
Ex vivo prime editing of patient haematopoietic stem cells rescues sickle-cell disease phenotypes after engraftment in mice, Nature Biomedical Engineering, 2023
Everette et al., David Liu Lab.
Diseases caused by genetic mutations may seem to be easy to treat and cure; just take the mutated DNA sequence and correct it, right? However, due to potential off-target effects, uninsured long-term stability, and complexities in delivery and specificity of the editing tools, the job is not as easily achievable as it seems. Or is it?
In the case of sickle-cell disease (SCD), a disease marked by crescent-shaped, or sickled, red blood cells caused by a point mutation in which DNA nucleotide adenine (‘A’) is improperly encoded as a thymine (‘T’) in the beta-globin gene, the only current FDA-approved cure involves allogeneic (from different individuals of the same species) hematopoietic stem cell (HSC) transplantation, a procedure in which the cells of a genetically similar donor are infused into a patient. However, many autologous (from the same individual) approaches for therapeutic manipulation of the implanted cells require double-stranded DNA breaks (DSBs), a serious damage that occurs when both DNA strands are severed at the same location, which causes uncontrolled combinations of mutations and abnormalities. Researchers circumvented this issue by using a tool called prime editing to replace a target sequence of DNA without using DSBs, and were able to confirm healthy phenotype and maintenance post-treatment, as well as assume high on-target efficiency to treat SCD.
What did these researchers do?
The researchers were able to develop an ex vivo (outside of the living body) delivery strategy that efficiently corrected mutated alleles (i.e. the incorrect DNA sequence that causes the disease) in SCD patient cells back to the wild-type allele (i.e. the correct DNA sequence that codes for healthy cells) using prime editing, a method that directly replaces a target DNA segment with a specified new sequence. In this method, cells could be edited, preserved, and engrafted into mice after 17 weeks with stable and high levels of on-target prime editing efficiency. They were able to visually confirm that treated cells exhibited less sickling behavior, strongly suggesting a viable therapeutic treatment for SCD patients. These discoveries help pioneer the therapeutic potential of prime editing and transplanting of HSCs.
Why is this important?
The researchers are pioneering in the realm of prime editing of HSCs for therapeutic purposes and were able to prove ample legitimacy within the experiments they conducted. Their findings suggest that prime editing in conjunction with transplantation of patient hematopoietic stem and progenitor cells (HSPCs) can be a viable one-time autologous treatment for SCD. SCD itself is a severe condition that introduces a multitude of complications for patients, ranging from immunodeficiency to acute and chronic pain and premature death. This research is significant in that it opens doorways for SCD patients to receive treatment that does not merely relieve symptoms, but visibly reverts sickled cells to a healthy state in a manner that is less risky and more robustly accurate than current treatments. Furthermore, this autologous approach offers a promising alternative to allogeneic transplantation treatment options, which are disadvantaged by increased risk of immune complications and procedural toxicities as well as difficulty in finding suitable donors, by using the patient’s own cells and therefore ensuring personalized therapies that bypass such drawbacks. Thus, such research holds the potential to revolutionize medicine through enabling precise genetic modifications in a safer and more effective manner. This strategy also holds a promising future regarding the development of treatments for other genetic diseases or engineered tissues for transplantation using prime editing.
How did the researchers do this?
The researchers designed a strategy using prime editing to revert the mutated SCD allele back to the wild-type allele in SCD patient donor cells. Prime editing involves using a fusion protein made of a Cas9 nickase (enzyme that makes a single-strand break) and a reverse transcriptase (enzyme that transcribes single-stranded RNA into DNA), and a guide RNA containing the desired edit encoded into its RNA sequence to direct the protein to a target location. This approach differs from that of the CRISPR-Cas9 technology, which uses Cas9 as molecular scissors to create double-stranded breaks (DSBs) in the DNA. Comparatively, prime editing allows for higher precision and versatility for making targeted edits within the genome than CRISPR-Cas9. Once the mutated SCD allele is reverted, both prime edited and untreated HSPCs from four SCD donors were transplanted into five mice to assess whether the edited cells could repopulate and maintain therapeutic levels of editing long-term in vivo (in a living organism). They found that the cells were stable over time, as they were able to maintain prime editing levels after 17 weeks and that 42% of engrafted human cells across four patients contained at least one wild-type allele, suggesting the robustness of the editing. One of the tell-tale phenotypes of SCD is the sickled appearance of cells in hypoxic conditions. By culturing the cells in such conditions, the researchers found that untreated control cells had an average of 63% sickled cells while treated cells from prime edited mice had 37% sickled cells. Little off-target editing was found, suggesting high on-target DNA specificity, as well (which is important, as off-target editing can lead to unanticipated side-effects).
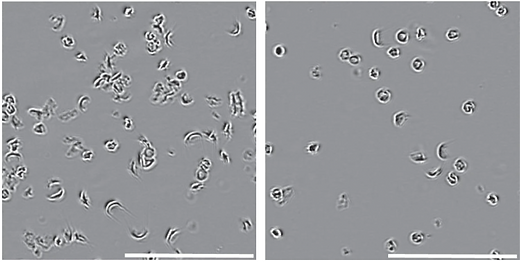
Untreated (left) SCD HSPCs exhibit more sickled phenotypes
than the treated (right) SCD HSPCs
What comes next?
Although this study demonstrates the successful application of ex vivo delivery for prime editing of HSPCs for SCD, further evaluation is still needed. To further optimize and validate this approach, conducting long-term studies to assess safety and efficacy and repeating these experiments in larger animal models can provide needed insight into the functionality in vivo. If properly validated, conducting clinical trials in human patients and considering this method as a viable treatment for SCD can be achieved. Cells derived from prime editing hold great implications in tissue engineering and further disease modeling or drug screening. Overall, successfully using prime editing in HSPCs to treat SCD marks a significant milestone; however, much research and development is needed to further advance this approach towards clinical translation and ultimate improvement of patient quality of life.